Can the Oxynoe bubble snail control invasive Caulerpa?
Invasive Seaweed and Grazing | Aven Zhuang
Caulerpa brachypus and Caulerpa parvifolia are two invasive seaweed species in New Zealand that have spread across many regions of the Hauraki Gulf and the Bay of Islands since they were first discovered in 2021. They form dense mats across the seafloor that smother benthic organisms, such as mussels, cockles, and scallops, causing major problems for cultural, recreational, and commercial activities. Recently, a bubble snail species, Oxynoe viridis, has been found feeding on the C. brachypus. Therefore, this research measured their grazing rate to explore its potential as a biological control for invasive Caulerpa.
Introduction
Caulerpa brachypus and Caulerpa parvifolia are two invasive seaweed species first detected at Aotea, Great Barrier Island in 2021 [1]. The two exotic species likely arrived from Australia or the Pacific Islands, with the accidental introduction occurring as a result of international yacht movements [1]. Caulerpa can reproduce asexually and spread via fragments that are broken off and capable of reattaching and growing on another surface [2]. Since 2021, these seaweeds have quickly dispersed and re-established in multiple new locations throughout the Hauraki Gulf and the Bay of Islands via the dispersal of fragments carried by currents and those attached to marine equipment [3].
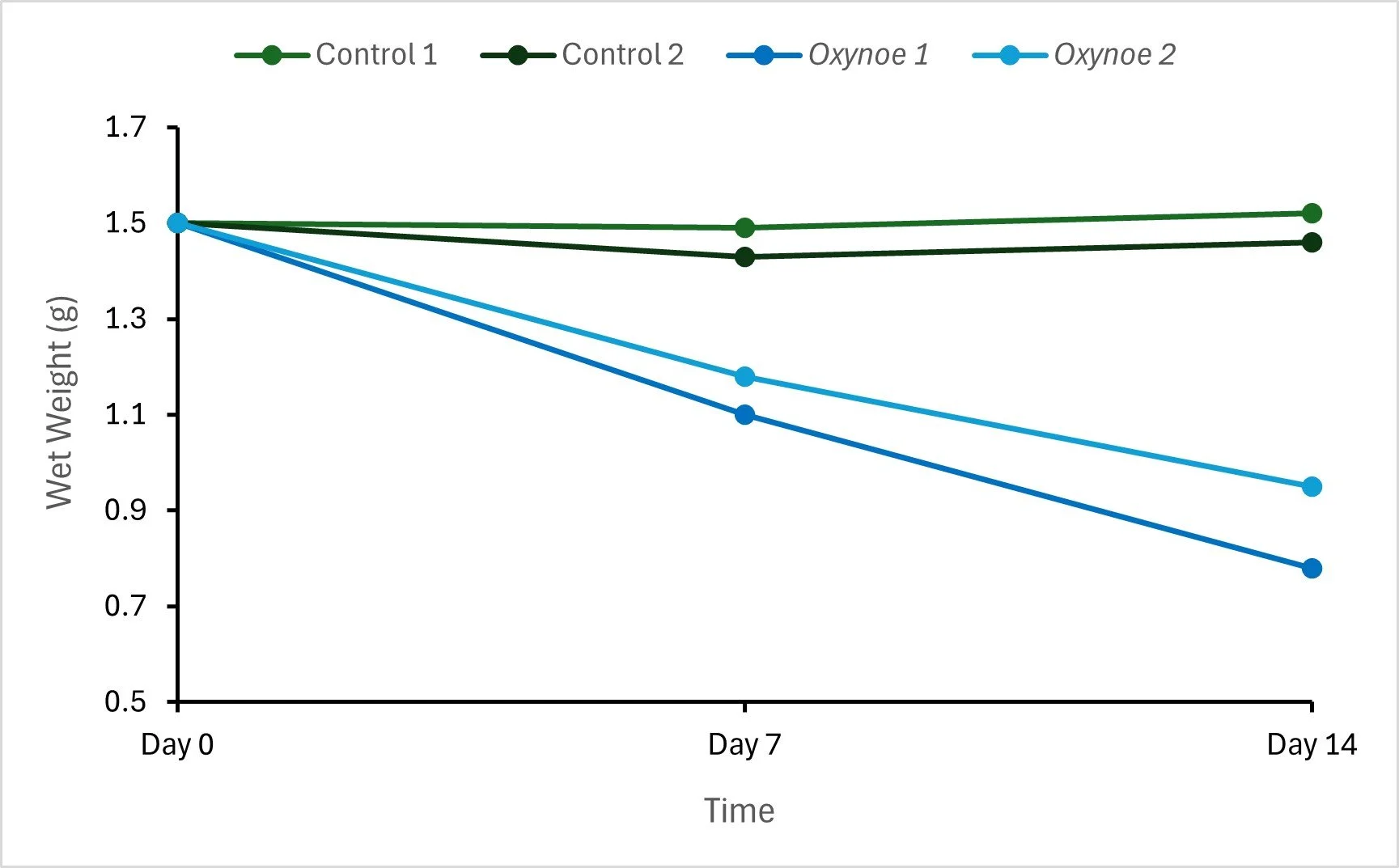
Figure 2: : The change in wet weight (g) of 1.50 g C. brachypus fragments with (Oxynoe) and without (control) grazing from O. viridis over two weeks (initial experiment).
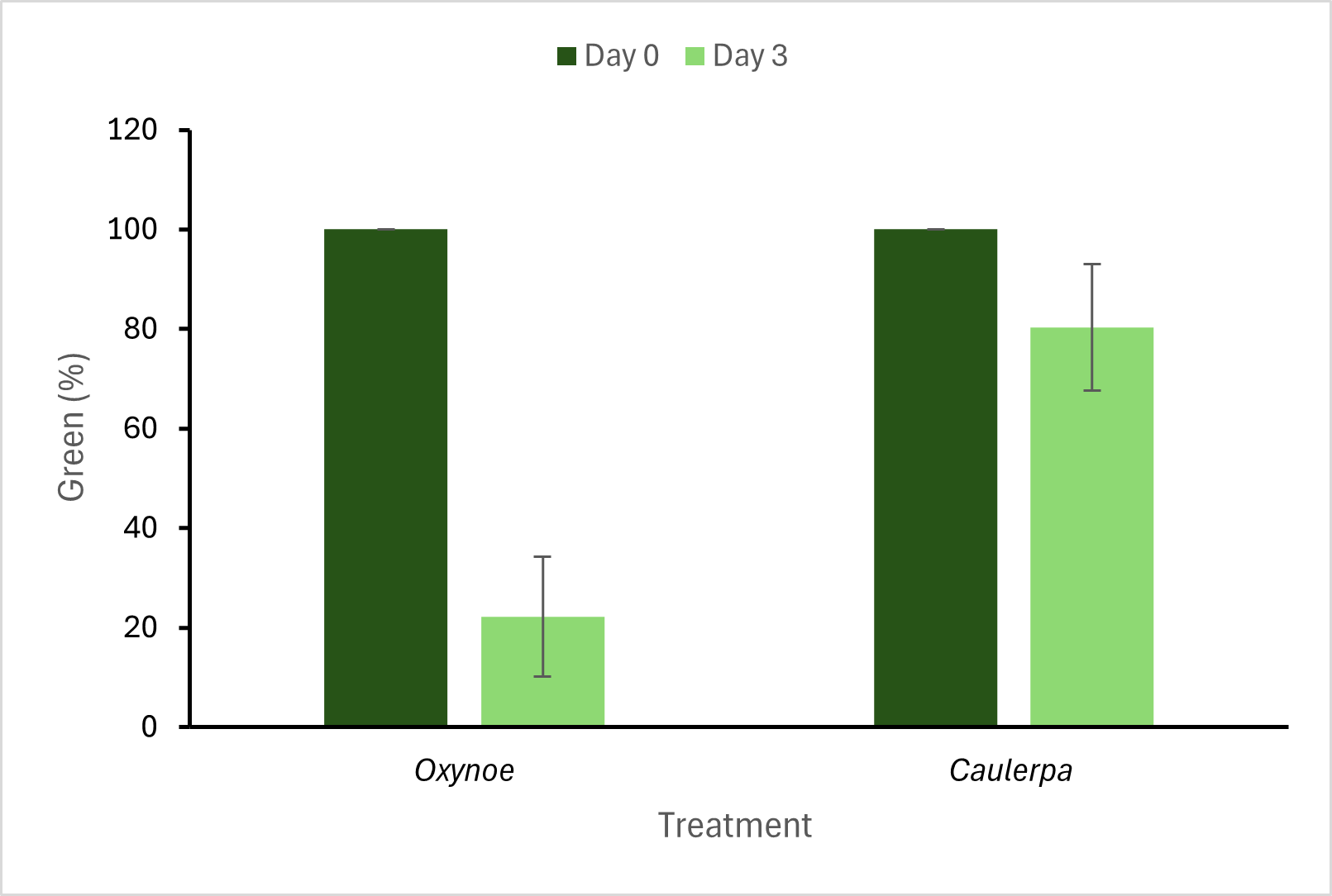
Figure 3: The green pigment percentage in C. brachypus fragments with and without grazing by O. viridis. The mean wet weight of C. brachypus fragments were 0.17 ± 0.01 g at the outset of the experiment.
Conclusion
Overall, O. viridis grazing is able to kill small fragments of C. brachypus and can potentially hinder its spread if large numbers were to be present among the Caulerpa. However, several knowledge gaps remain, including the larval development of O. viridis, its ability to be reared in captivity, and its feeding preferences among other Caulerpa species found in New Zealand. Therefore, future research on understanding these factors is essential to evaluate its potential as a biocontrol for managing invasive Caulerpa excursions. In conclusion, this project assessed the grazing potential of O. viridis on C. brachypus and provided some initial insights on the viability of its utility as a biocontrol agent.
Figure 1: Oxynoe viridis found in the Caulerpa samples from Aotea - Great Barrier Island. Photo credit: Richard Taylor.
The laboratory pair of O. viridis have frequently produced copious quantities of egg masses, indicating they are also likely to be simultaneous hermaphrodites. Thus, the observations of the O. viridis (feeding and reproduction) suggest they have potential to be raised in large numbers in captivity to control invasive Caulerpa in areas where other tools may not be suitable.
Currently, there are very limited studies regarding the O. viridis feeding rates, larval rearing, and viability of field deployment. Hence, this project aimed to find the grazing rate of O. viridis on C. brachypus (the more dense, resilient species) to determine its suitability to be used as a biocontrol for invasive Caulerpa.
Methods
The Caulerpa samples and O. viridis used in the experiments were collected from Aotea - Great Barrier Island. The initial experiment measured the grazing rates of O. viridis by assessing the decrease in wet weight of C. brachypus fragments (including thalli, stolon and rhizoids) over two weeks. Each O. viridis snail (n = 2) was housed in a 300 ml clean cylindrical polycarbonate container containing 250 ml of 1 µm filtered, ultraviolet sterilised seawater (35 ppt salinity) along with 1.50 g of C. brachypus fragments. Two additional containers, identical in setup but without O. viridis, were used as controls. Overhead lighting was provided for the containers using Aqua One Stripglo Plant LED Lights at 25 µmol m-2 s-1 over 24h. The Caulerpa fragments were weighed at the start (day 0) and subsequently at weekly intervals (i.e., days 7 and 14). Before weighing, excess water was gently removed using a paper towel to ensure consistency. The seawater in the containers was changed weekly after weighing the fragments, and the water temperature was constant at 18.5 ºC.
In the second experiment, grazing was measured using ImageJ by comparing the decrease in green pigmentation (chlorophyll content) in C. brachypus fragments over two days. Digital photos of C. brachypus fragments were taken at the outset of the experiment (day 0) and after two days of grazing (day 3), on a glass petri dish next to a 15 cm ruler for scale. The digital photographs of the fragments were imported into the image analysis software ImageJ [13], and the type was converted to [8-bit] grey scale. Using the [Auto Threshold] function, the photographs were turned black and white, in which the white sections within the dish corresponded to the green pigment of the fragment. The scale was set using a 10 mm reference from the ruler in the image. Then, the total green area (mm²) and percentage green were calculated by circling the area around the fragment and using the [Analyse Particles] function. The change in wet weight of the fragments was also recorded to compare with the grazing rate from the previous experiment. Due to issues with the collection of Oxynoe snails, the sample size used in the experiments was small, so the experiment was conducted twice for replication and to capture more of the variation in grazing. In total, the green pigmentation of 12 sets of Caulerpa fragments (0.17 ± 0.01 g) were assessed, where three O. viridis snails were used for grazing (lengths: 24.5mm, 24.5mm and 19.8 mm) and three without as controls per experiment repeat.
Results
The wet weight of the Caulerpa fragments without grazing from O. viridis changed slightly over two weeks by, on average, 0.01 ± 0.02 g of the starting wet weight. In contrast, the wet weight of fragments with O. viridis grazing declined each week, where the decrease in wet weight of the fragments (or grazing rate) was, on average, 0.05 g d-1 (Fig. 2). By the end of the two weeks, there were still some parts of the fragments that were green, indicating it will likely require more than two weeks for a single O. viridis to fully consume 1.50 g of Caulerpa fragments. Hence, the second experiment was shorter and used fewer fragments, allowing for more precise measurements of O. viridis grazing.
In the second experiment, there was, on average, an 80 ± 12.7 % decrease in the area of the C. brachypus fragment retaining green colouration (chlorophyll) after two days of grazing from O. viridis. The mean decrease in wet weight of the same fragments over this period was 0.12 ± 0.02 g. The C. brachypus fragments without any grazing showed a 20 ± 12 % decrease in green colouration and 0.006 g ± 0.005 g mean wet weight, likely due to handling stress (Fig. 3). The mean grazing rate measured using the wet weight of the fragments in this experiment was 0.06 ± 0.005 g d-1.
Discussion
The first experiment indicated that it required approximately two weeks for a single O. viridis to graze away most of the 1.50 g C. brachypus fragment. This is equivalent to a small clump of the seaweed covering approximately 16 cm2 of seafloor. Given that around 44 ha of the seafloor in Okupu Bay, Aotea, is covered in Caulerpa [7], the observed grazing rates of O. viridis may not be high enough to majorly impact the Caulerpa cover unless they are present in very large numbers.
The second experiment showed that, on average, a single O. viridis snail can consume approximately 80% of the chlorophyll content in 0.17 g of C. brachypus fragments over two days. The loss of chlorophyll due to grazing caused the affected portions of the algal tissue to die, turning white and resembling fragments treated with bleach (MPI protocols). A previous study has found that Caulerpa taxifolia thalli (5 cm) ceased to grow when there were more than 15 incisions ·cm–1 made by Oxynoe olivacea [14]. The fragments after grazing in this experiment were moved into new containers without O. viridis and observations indicated that they did not seem to exhibit growth after a week. Thus, the 80% decrease in chlorophyll content likely exceeded the threshold for Caulerpa growth.
A study found that O. viridis has strong preferences for Caulerpa species with low secondary metabolite concentrations, such as C. racemosa and C. lentillifera [10]. However, those Caulerpa species in that study are not present in New Zealand, and this project solely looked at the grazing rate of O. viridis on C. brachypus fragments. Furthermore, seawater temperature can be a factor that can affect the grazing rates of O. viridis. For example, a study has found the mean number of incisions made by O. olivacea on C. taxifolia thalli almost doubled at and above 22 °C compared to those observed between 13 and 19 °C [14]. Thus, future research should investigate how temperature may affect the grazing rates of O. viridis and its feeding preferences among Caulerpa species found in New Zealand (i.e. C. parvifolia and native species). This would be crucial in determining its suitability as a biocontrol tool since artificially enhanced populations of the snails used for biocontrol could have negative consequences for populations of native Caulerpa species.
Due to difficulties and delays in obtaining additional O. viridis snails for the laboratory, the second experiment had a small sample size and limited replication. As a result, the observed grazing rates may not have captured sufficient variation. Currently, the grazing rates in this study provide an initial indication of their grazing rates. Hence, the future studies will have a larger sample size or increased experiment repetition to improve the reliability of the findings, and better capture variability in grazing.
Glossary
Augmentative biocontrol: the use of native or naturally occurring species to control pest species and does not rely on introduced species to be effective [15 -16].
The dense exotic Caulerpa mats have been observed to smother benthic organisms, such as mussels, cockles, and scallops [4]. The exclusion of native marine life in benthic habitats can impact cultural, recreational, and commercial activities. For instance, there has been limited gathering of molluscs and crustaceans at Okupu/Blind Bay due to the spread of invasive Caulerpa [4]. Furthermore, benthic shellfish beds are important in ecosystems because they help water filtration and nutrient cycling and can provide a complex structural habitat supporting diverse invertebrates and fishes [5-6]. Thus, the decline in shellfish beds from the spread of Caulerpa can negatively impact the ecosystem and reduce overall biodiversity.
The current approach by the Ministry for Primary Industries (MPI) for managing exotic Caulerpa in New Zealand is to slow the spread within the existing infested areas and prevent spread into new areas [3]. Various Caulerpa eradication and control methods have been tested, such as chemical (salt and chlorine) and physical approaches (suction dredging, benthic mats, UV light, and hand removals). Whilst some have shown promise, some tools are often expensive, environmentally limited (depth and substrate dependant), and can unintentionally affect non-target species and habitats [7]. Therefore, a potential alternative method is augmentative biological control, specifically using a native bubble snail species, Oxynoe viridis, which are specialist feeders on Caulerpa.
Oxynoe is a group of shelled sacoglossans (Gastropoda: Heterobranchia), snails of which many species of the genus can feed on Caulerpa species despite the anti-grazing compounds that are often present in the seaweed [8-10]. Oxynoeviridis is a species native to the Indo-Pacific region [11]. During 2024, three O. viridis were found within the laboratory Caulerpa samples in the School of Biological Sciences. Initial close observations of the snails in captivity showed they actively feed by sucking the cellular contents out of the algae’s tissue, resulting in its death. A previous study found that Oxynoe olivacea are simultaneous hermaphrodites (have both male and female sex organs) and can alternate between the sexual roles [12].
Acknowledgments
I’d like to thank my supervisor Andrew Jeffs for giving me the opportunity to work on the amazing summer research project. Thank you to Richard Taylor for taking the beautiful photo of the Oxynoe bubble snail.
[1] Ministry for Primary Industries (MPI). (2024a). Exotic Caulerpa seaweeds in New Zealand. https://www.mpi.govt.nz/ biosecurity/exotic-pests-and-diseases-innew-zealand/pests-and-diseases-underresponse/exotic-caulerpa-seaweedscaulerpa-brachypus-and-caulerpaparvifolia-in-new-zealand/
[2] C. M. Smith and L. J. Walters, “Fragmentation as a Strategy for Caulerpa Species: Fates of Fragments and Implications for Management of an Invasive Weed,” Marine Ecology, vol. 20, no. 3–4, pp. 307–319, Dec. 1999, doi:https://doi.org/10.1046/j.1439-0485.1999.2034079.x.
[3] Ministry for Primary Industries. (2024b). Technical advice on a longterm management strategy for exotic Caulerpa. https://www.mpi. govt.nz/biosecurity/exotic-pests-and-diseases-in-new-zealand/ pests-and-diseases-under-response/exotic-caulerpa-seaweedscaulerpa-brachypus-and-caulerpa-parvifolia-in-new-zealand/
[4] Ministry for Primary Industries (MPI). (2021). Caulerpa – Great Barrier Island 2021 Biosecurity Response. https://www.mpi.govt. nz/dmsdocument/46393-Caulerpa-Great-Barrier-Island-2021- Biosecurity-Response-Fact-sheet
[5] J. A. Commito, S. Como, B. M. Grupe, and W. E. Dow, “Species diversity in the soft-bottom intertidal zone: Biogenic structure, sediment, and macrofauna across mussel bed spatial scales,” Journal of Experimental Marine Biology and Ecology, vol. 366, no. 1–2, pp. 70– 81, Nov. 2008, doi: https://doi.org/10.1016/j.jembe.2008.07.010
[6] I. McLeod, D. Parsons, M. Morrison, S. Van Dijken, and R. Taylor, “Mussel reefs on soft sediments: a severely reduced but important habitat for macroinvertebrates and fishes in New Zealand,” New Zealand Journal of Marine and Freshwater Research, vol. 48, no. 1, pp. 48–59, Sep. 2013, doi: https://doi.org/10.1080/00288330.2013.8 34831.
[7] B. Scott, “THE ANATOMY OF AN INVASION EXOTIC CAULERPA AT AOTEA GREAT BARRIER ISLAND,” 2024. Accessed: Mar. 02, 2025. [Online]. Available: https://www.gbiet.org/s/The-Anatomy-ofInvasion_Caulerpa-on-Aotea-Great-Barrier-Island-v10.pdf
[8] P. Gianguzza, L. Airoldi, R. Chemello, C. D. Todd, and S. Riggio, “Feeding preferences of Oxynoe olivacea (Opisthobranchia: Sacoglossa) among three Caulerpa species.,” vol. 68, no. 3, 2002. https://www. academia.edu/download/48150754/289.full.pdf
[9] P. J. Krug, J. S. Berriman, and Á. Valdés, “Phylogenetic systematics of the shelled sea slug genus Oxynoe Rafinesque, 1814 (Heterobranchia : Sacoglossa), with integrative descriptions of seven new species,” Invertebrate Systematics, vol. 32, no. 4, p. 950, 2018, doi: https://doi. org/10.1071/is17080.
[10] F. Baumgartner, C. Motti, R. de Nys, and N. Paul, “Feeding preferences and host associations of specialist marine herbivores align with quantitative variation in seaweed secondary metabolites,” Marine Ecology Progress Series, vol. 396, pp. 1–12, Dec. 2009, doi: https:// doi.org/10.3354/meps08359.
[11] “Oxynoe viridis,” iNaturalist, 2025. https://www.inaturalist.org/ taxa/380373-Oxynoe-viridis (accessed Mar. 02, 2025).
[12] P. Gianguzza, F. Badalamenti, J. K. R, R. Chemello, S. Cannicci, and S. Riggio, “Body Size and Mating Strategies in the Simultaneous Hermaphrodite Oxynoe olivacea (Mollusca, Opisthobranchia, Sacoglossa),” Functional Ecology, vol. 18, no. 6, pp. 899–906, 2004, doi: https://doi.org/10.2307/3599118.
[13] C. A. Schneider, W. S. Rasband, and K. W. Eliceiri, “NIH Image to ImageJ: 25 Years of Image Analysis,” Nature Methods, vol. 9, no. 7, pp. 671–675, Jun. 2012, doi: https://doi.org/10.1038/nmeth.2089.
[14] T. Thibaut and A. Meinesz, “Are the Mediterranean ascoglossan molluscs Oxynoe olivacea and Lobiger serradifalci suitable agents for a biological control against the invading tropical alga Caulerpa taxifolia?,” Comptes Rendus de l’Académie des Sciences - Series III - Sciences de la Vie, vol. 323, no. 5, pp. 477–488, May 2000, doi: https://doi.org/10.1016/s0764- 4469(00)00151-7.
[15] J. L. Capinera, Encyclopedia of Entomology. Springer Science & Business Media, 2008.
[16] J. A. Stenberg et al., “When is it biological control? A framework of definitions, mechanisms, and classifications,” Journal of Pest Science, vol. 94, no. 3, pp. 665–676, Mar. 2021, doi: https://doi.org/10.1007/ s10340-021-01354-7.
Aven has completed his BAdvSci(Hons) in Marine Science. His dissertation focussed on how the growth of exotic Caulerpa fragments varied at different temperatures, salinity, and light levels. Building on this foundation, Aven is aiming to continue researching Caulerpa at the PhD level, focusing on its ecological and social impacts.